Changes of Matter
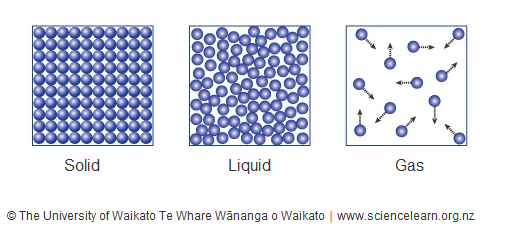
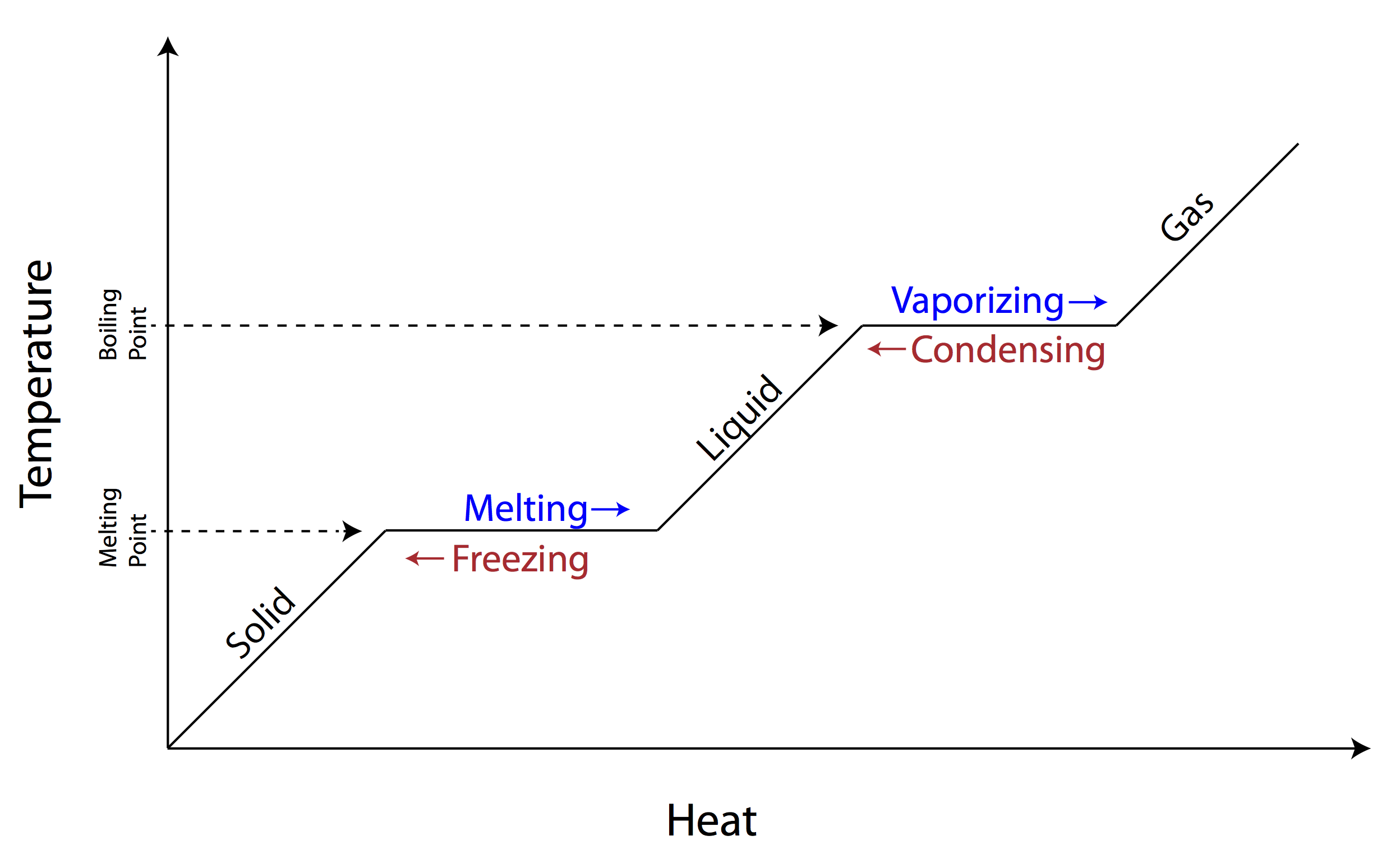
The changes of matter involve transformations between the four fundamental states: solid, liquid, gas, and plasma. These changes are driven by alterations in temperature and pressure, leading to various phase transitions. Vaporization is the process by which a substance changes from a liquid to a gas, such as water boiling to form steam. Condensation is the opposite process, where a gas changes back into a liquid, like steam condensing to form water droplets. Boiling is a specific type of vaporization that occurs when a liquid reaches its boiling point, resulting in the rapid formation of bubbles and conversion to a gas.
Changes of Matter
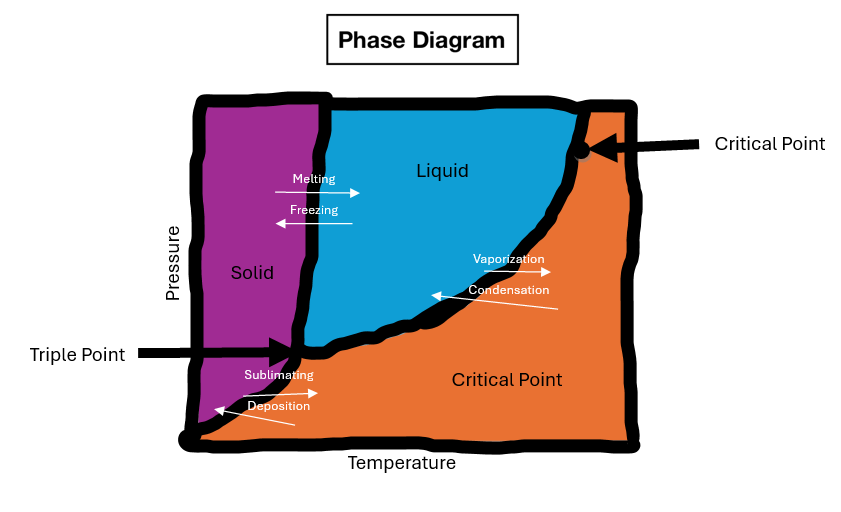
Freezing is the process in which a liquid changes to a solid, such as water freezing into ice. Sublimation is the direct transition of a substance from a solid to a gas, skipping the liquid phase, as seen with dry ice (solid carbon dioxide) turning into carbon dioxide gas. Deposition is the reverse process, where a gas changes directly into a solid, like frost forming on a cold surface. Ionization occurs when a gas becomes ionized, forming a plasma by losing or gaining electrons. Recombination is the opposite process, where ions recombine to form neutral atoms or molecules, leading to the reformation of a gas from a plasma.
Understanding Matter's Transformations
Understanding these changes of matter is crucial in various scientific and industrial contexts. For example, in the field of materials science, knowledge of phase transitions is essential for designing materials with specific properties. In atmospheric science, an understanding of phase changes is crucial for predicting weather patterns and studying climate change. In engineering, the ability to control phase transitions is vital for designing efficient processes and technologies, such as refrigeration systems and chemical reactors. Overall, the changes of matter are fundamental to our understanding of the physical world and play a significant role in shaping our technological advancements and scientific discoveries.